Light exposure regulates how two kinds of fat cells (adipocytes) work together to produce the raw materials that all other cells use for energy, according to a new study conducted in mice.
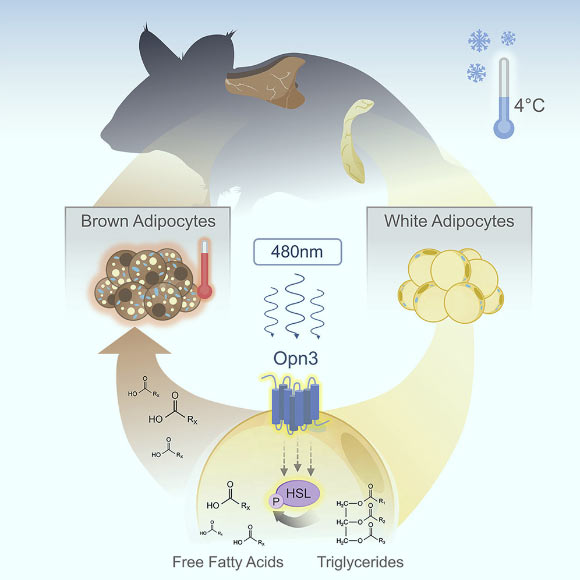
White fat cells (adipocytes) activate the lipolysis pathway to produce the free fatty acids that are used as heating fuel by brown adipose tissue. Nayak et al show that OPN3 is required for blue-light-enhanced activation of the lipolysis pathway. This explains the low body temperature of OPN3 mutant mice. Image credit: Nayak et al, doi: 10.1016/j.celrep.2019.12.043.
“Our bodies evolved over the years under the Sun’s light, including developing light-sensing genes called opsins,” said Dr. Richard Lang, a developmental biologist at the University of Cincinnati and Cincinnati Children’s Hospital Medical Center.
“But now we live so much of our days under artificial light, which does not provide the full spectrum of light we all get from the Sun.”
“This study represents a significant change in the way we view the effects of light on our bodies.”
In the research, Dr. Lang and colleagues studied how mice respond when exposed to chilly temperatures — about 40 degrees Fahrenheit (4.4 degrees Celsius).
They already knew that mice, much like humans, use both a shivering response and an internal fat-burning response to heat themselves.
Deeper analysis revealed that the internal heating process is compromised in the absence of the gene encephalopsin (OPN3) and exposure specifically to a 480-nm wavelength of blue light. This wavelength is a natural part of sunlight but occurs only in low levels in most artificial light.
When the light exposure occurs, OPN3 prompts white fat cells to release fatty acids into the bloodstream. Various types of cells can use these fatty acids as energy to fuel their activities. But brown fat literally burns the fatty acids — in a process called oxidation — to generate heat that warms up the chilly mice.
When mice were bred to lack the OPN3 gene, they failed to warm up as much as other mice when placed in chilly conditions.
But surprisingly, even mice that had the correct gene failed to warm up when they exposed to light that lacked the blue wavelength.
These data prompted the researchers to conclude that sunlight is required for normal energy metabolism; at least in mice.
While they strongly suspect that a similar light-dependent metabolic pathway exists in humans, they need to complete another series of experiments to prove it.
“If the light-OPN3 adipocyte pathway exists in humans, there are potentially broad implications for human health,” they said.
“Our modern lifestyle subjects us to unnatural lighting spectra, exposure to light at night, shift work, and jet lag, all of which result in metabolic disruption.”
“Based on the current findings, it is possible that insufficient stimulation of the light-OPN3 adipocyte pathway is part of an explanation for the prevalence of metabolic deregulation in industrialized nations where unnatural lighting has become the norm.”
Someday, in theory, ‘light therapy’ could become a method for preventing metabolic syndrome from developing into diabetes.
“Replacing indoor lights with better, full-spectrum lighting systems also could improve public health,” Dr. Lang said.
“However, more study is needed to pin down the potential therapeutic value of light therapy.”
“For now, however, if people want to take anything personal away from this, you likely can’t go wrong by spending more time outside,” Dr. Lang said.
The study was published in the journal Cell Reports.
_____
Gowri Nayak et al. 2020. Adaptive Thermogenesis in Mice is Enhanced by Opsin 3-Dependent Adipocyte Light Sensing. Cell Reports 30 (3): 672-686; doi: 10.1016/j.celrep.2019.12.043







